Modern CMMS solutions make tracking manufacturing Standard Operating Procedures compliance easy–from OSHA requirements to quality assurance and product standards to planned maintenance and manufacturing regulations. With digital audits and checklists, management can easily track production processes and work orders and standardize business processes.
With Computerized Maintenance Management Software or CMMS, monitoring compliance is simple. Manufacturers can automate SOP completion and preventive maintenance operations to enhance the quality of products. Critical data, metrics, and checklists are easily accessible for inspectors and managers.
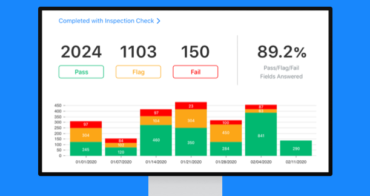
Digital manufacturing Standard Operating Procedures help teams manage and track audits and adhere to laws and regulations and quality controls. Enterprise asset management is easy to keep track of.
Across a company, facility management, maintenance teams, and management teams use SOP to meet state and federal laws and regulations.
And with the time saved by automating SOP comes more time for innovation. More preventive maintenance by team members on a piece of equipment means less reactive maintenance. More time to improve products or services.
Manufacturers can manage maintenance and demonstrate compliance regulations through manufacturing SOP that promote quality assurance and continuous improvement.
Preventive maintenance plans and Standard Operating Procedures enhance a company’s ability to produce and market products at an extraordinary rate, especially against competition from international markets.
With CMMS, companies stay on top of planned and preventive maintenance schedules SOP.
Manufacturing SOP and Compliance
Going digital with Standard Operating Procedures improves compliance.
Manufacturers deal with many areas of compliance, including laws and regulations, product safety, and health and environmental regulations.
Quality assurance (QA) plans to ensure that manufactured products meet regulatory and quality standards.

Quality assurance plans are an important part of a manufacturing company.
The International Organization for Standardization (ISO) developed ISO 9000 to define a “quality management system.” ISO 9000 is now used across manufacturing facilities in the US and globally.
Government regulations have increased with the emergence of global markets. The FDA’s Quality Policy states that:
Management with executive responsibility shall establish its policy and objectives for, and commitment to, product quality. Management with executive responsibility shall ensure that the quality policy is understood, implemented, and maintained at all levels of the organization.
OSHA inspectors look for anything that can cause danger or bodily harm and impact an employee’s life on or off the job. OSHA’s General Duty Clause enforces worker health and safety.
Manufacturing Standard Operating Procedures and Tracking
Manufacturing compliance requires tracking changes. Tracking information and decisions is easier when the tracking is digital, especially when suppliers and new outsourcing are involved.
Compliance in the manufacturing process increases when data is collected and analyzed in real-time.
With tracked Standard Operating Procedures data, manufacturers and inspectors can carry-out critical audits and regulatory checks on the factory floor.
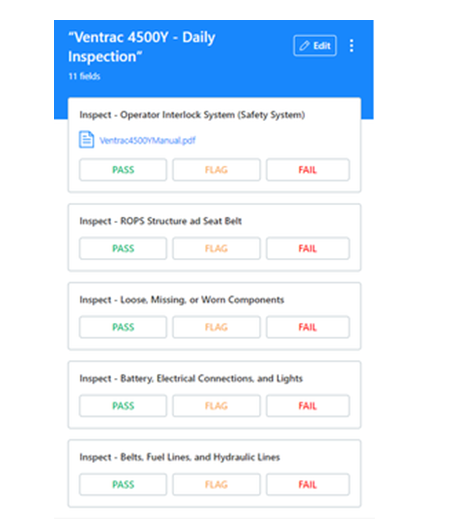
Digitizing Standard Operating Procedures help achieve greater efficiency in meeting preventive maintenance, reactive maintenance, quality assurance, regulatory compliance, and work orders.
CMMS solutions provide on-the-spot document management to help pass inspections and head off potential legal problems. Mobile forms speed up the transfer of information without sacrificing accuracy. Management Standard Operating Procedures outline preventive, reactive, and predictive maintenance with compliance checklists.
Online data collection reduces the time needed to perform audits.
Quality management plans and SOP provide opportunities to locate inefficiency and downtime in the manufacturing process. SOP also provides evidence of regulatory compliance on the factory and warehouse floor. Online SOP templates can help build an organizational culture of excellence.
Online dashboards and checklists can be shared with other people to improve work and communication between all levels of a team. This also reduces the risk of non-compliance and improves supply chain efficiencies.
In summary, now is the time to digitize your SOP and workflows. Modern solutions have never been more accessible or insightful.